INNOVATION FOR
EARLY DETECTION
LifeOS Genomics is dedicated to
building the most uncompromising
liquid biopsy platform
Genetic Testing of Liquid Biopsy on Late Stage Lung Cancer for 3rd Generation Medicine Treatment
Breakthrough in Testing for Lung Cancer Patients! The collaboration between National Taiwan University’s Genomic Lab and LifeOS Genomics Co., Ltd. has resulted in the development of laboratory-developed tests (LDTs) for T790M gene detection.
Recently, these testing projects were announced as approved by the Taiwan Food and Drug Administration (TFDA). In the future, once hospitals and laboratories sign agreements and report to the Ministry of Health and Welfare, these technologies can be applied in clinical settings.
Experts indicate that approximately half of lung cancer patients have EGFR gene mutations. If resistance develops after using first and second-generation drugs, T790M testing can be performed through blood samples, replacing the live tissue biopsy process that may lead to complications such as pneumothorax or hemothorax. This approach facilitates the smooth usage of third-generation drugs for patients in the advanced stages.
Lung cancer has the highest mortality rate in Taiwan. According to statistics, over 50% of patients have epidermal growth factor receptor (EGFR) mutations, responding well to EGFR-TKIs. However, within a year, 60% of patients may develop resistance due to the secondary mutation EGFR T790M, requiring third-generation EGFR-TKI treatment. Director Yu Song-liang of the Department of Medical Laboratory Science and Biotechnology at National Taiwan University explains that currently, end-stage lung cancer patients are covered by health insurance for the direct use of first and second-generation drugs.
However, meeting specific conditions is necessary for free access to third-generation drugs, requiring T790M gene testing.
The current method of gene testing for lung cancer involves live tissue biopsy, as mentioned by Yu Song-liang. This biopsy method requires a needle and is guided by ultrasound. During the process, patients may experience complications such as pneumothorax or hemothorax, requiring immediate medical attention. Not everyone is willing to undergo live tissue biopsy. In comparison, the LDTs from the National Taiwan University lab only require a blood sample, with minimal side effects. The recent FDA approval allows for the use of these tests once hospitals and labs sign agreements and report to the Ministry of Health and Welfare. Since there is no health insurance coverage, patients may need to self-fund around NT$10,000.
Yu Song-liang emphasizes the significance of the approved LDTs, marking the first step in developing external testing reagents. This technology, developed independently in Taiwan, reduces costs, providing a fast and highly accurate method. In the future, if different biological markers for various diseases are identified, this technology can be applied to early screening, medication guidance, and postoperative recurrence monitoring for various cancers or other chronic diseases, representing a major breakthrough in precision healthcare in Taiwan.
We Are Platform Builders.
We Are Clinical Solution Engineers.
LifeOS is developing better clinical solutions for complex genetic diseases using liquid biopsy.
Introducing the QLoci™ System
Powerful true multiplexing technology based on the digital PCR process.
Clinical testing platform for high sensitivity screening and diagnostics applications.




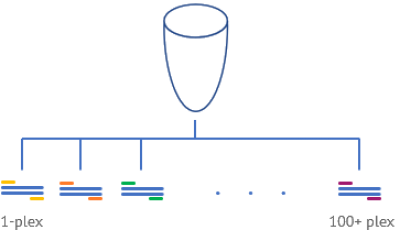
Multiplex
Look at 100+ targets with multiplex digital PCR.
Automation
From sample to result.
Applicable
Absolute quantitation for dynamic applications.
Optimized for liquid biopsies.
Bringing liquid biopsy into the forefront of genetic testing and healthcare.
LifeOS’ QLoci system offers 0.01% sensitivity assay panels with sample-to-result workflows.
mdPCR™
Our patented mdPCR technology is a fast and reliable way to quantify large numbers of targets of interest in a single sample of nucleic acid.
Coupling digital PCR principles with a molecular coding strategy, mdPCR affords both the sensitivity of dPCR and the capability of highly multiplexed methods, while providing reliable quantitation results.
System Workflow

1. DNA Preparation
Extract and pre-amplify your samples in the QLoci SP24 Instrument or a conventional thermal cycler.

2. Sample-to-Result
Reagents mixing, partitioning, data analysis, analyzing, and reading all on the QLoci md1000 Analyzer.
No more stations and modules, think intelligent automation.
QLoci™ Assay Panels
QLoci Assay Panels are closed system testing panels tailored towards specific clinical applications. Assay panels are made ready-to-use and clinically validated.
QLoci™ NIPT Assay Panel
The only PCR-based assay to assess chromosomal aneuploidy T21, T18, T13, X, Y.

Collaboration Breeds Success
We offer an unique platform for medical laboratories, researchers, and hospital systems who think bigger for their clinical applications.
Join LifeOS and our partners in adopting the mdPCR technology in genetic testing, cancer screening, companion diagnostics, and infectious disease monitoring.
Interested in adopting the QLoci System for your biomarkers?